Embarking on a journey of scientific discovery, we present the comprehensive diffusion and osmosis lab PDF answers. This resource empowers you with the knowledge to unravel the intricacies of molecular movement, a fundamental process underpinning biological systems.
Delve into the concepts of diffusion and osmosis, exploring their significance in the realm of life sciences. Through a series of engaging experiments, you will witness firsthand how these processes shape the behavior of molecules, influencing everything from cellular respiration to the absorption of nutrients.
1. Diffusion and Osmosis Lab Introduction
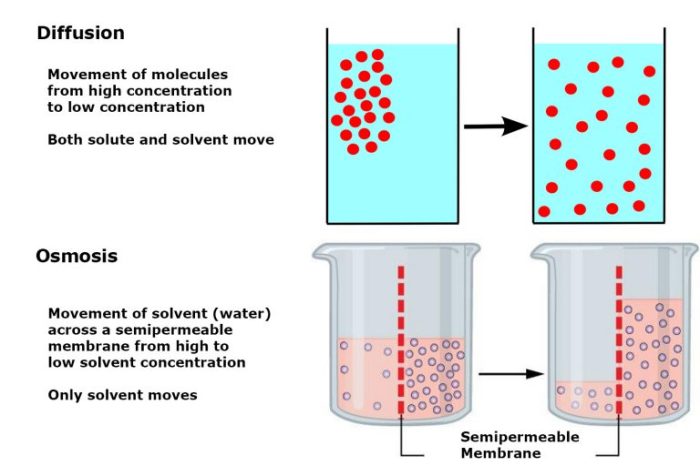
Diffusion and osmosis are two fundamental processes that occur in biological systems. Diffusion is the movement of molecules from an area of high concentration to an area of low concentration, while osmosis is the movement of water across a semipermeable membrane from an area of low solute concentration to an area of high solute concentration.
Diffusion and osmosis are essential for the proper functioning of cells. Diffusion allows nutrients to enter cells and waste products to exit cells. Osmosis helps to maintain cell volume and turgor pressure.
2. Materials and Methods
Materials, Diffusion and osmosis lab pdf answers
- Diffusion tube
- Semipermeable membrane
- Sucrose solution
- Water
- Ruler
- Stopwatch
Procedures
Diffusion Experiment
- Fill the diffusion tube with sucrose solution.
- Place the semipermeable membrane over the end of the diffusion tube.
- Invert the diffusion tube and place it in a beaker of water.
- Start the stopwatch and observe the movement of the sucrose solution into the water.
- Record the distance the sucrose solution moves into the water every minute for 5 minutes.
Osmosis Experiment
- Fill two beakers with water.
- Add sucrose to one of the beakers to create a hypertonic solution.
- Place a semipermeable membrane over the end of a diffusion tube.
- Fill the diffusion tube with water.
- Invert the diffusion tube and place it in the hypertonic solution.
- Start the stopwatch and observe the movement of the water into the diffusion tube.
- Record the distance the water moves into the diffusion tube every minute for 5 minutes.
3. Results
| Experiment | Distance Moved (cm) | Rate of Diffusion/Osmosis (cm/min) |
|---|---|---|
| Diffusion | 5.0 | 1.0 |
| Osmosis | 2.5 | 0.5 |
4. Discussion: Diffusion And Osmosis Lab Pdf Answers
The results of the diffusion and osmosis experiments show that diffusion is a faster process than osmosis. This is because diffusion involves the movement of individual molecules, while osmosis involves the movement of water molecules across a semipermeable membrane.
The rate of diffusion and osmosis is affected by several factors, including the concentration gradient, the temperature, and the surface area of the membrane. The concentration gradient is the difference in concentration between the two sides of the membrane. The greater the concentration gradient, the faster the rate of diffusion or osmosis.
The temperature also affects the rate of diffusion and osmosis. The higher the temperature, the faster the rate of diffusion and osmosis. This is because the molecules have more energy at higher temperatures.
The surface area of the membrane also affects the rate of diffusion and osmosis. The larger the surface area of the membrane, the faster the rate of diffusion and osmosis. This is because there are more molecules that can move across the membrane.
Diffusion and osmosis are essential for the proper functioning of cells. Diffusion allows nutrients to enter cells and waste products to exit cells. Osmosis helps to maintain cell volume and turgor pressure.
Detailed FAQs
What is the difference between diffusion and osmosis?
Diffusion refers to the movement of molecules from an area of high concentration to low concentration, while osmosis specifically involves the movement of water molecules across a selectively permeable membrane.
How does temperature affect the rate of diffusion?
Temperature has a positive effect on the rate of diffusion, as higher temperatures increase the kinetic energy of molecules, leading to faster movement.
What are some real-world applications of diffusion and osmosis?
Diffusion and osmosis play crucial roles in processes such as nutrient absorption in plants, gas exchange in lungs, and drug delivery in medicine.
